Approach Considerations
Arthrocentesis of the affected joint is mandatory for all patients with new-onset acute monoarthritis and is very strongly recommended for those with recurrent attacks whose diagnosis has never been proved by microscopic visualization of crystals. Tophi also may be aspirated for crystal analysis under polarizing microscopy.
A prior history of gout or pseudogout does not rule out the possibility of acute septic arthritis. In fact, the latter is more common in patients with a history of crystal-induced arthritis. Septic arthritis must be diagnosed and treated promptly, because irreversible damage can occur within 4-6 hours and the joint can be completely destroyed within 24-48 hours.
Send joint fluid for fluid analysis, including cell count and differential, Gram stain, culture and sensitivity, and microscopic analysis for crystals. If crystals are seen, their shape and appearance under polarized light are diagnostic.
In gout, crystals of monosodium urate (MSU) appear as needle-shaped intracellular and extracellular crystals. When examined with a polarizing filter and red compensator filter, they are yellow when aligned parallel to the slow axis of the red compensator but turn blue when aligned across the direction of polarization (ie, they exhibit negative birefringence). Negatively birefringent urate crystals are seen on polarizing examination in 85% of specimens.
Microscopic analysis in pseudogout shows calcium pyrophosphate (CPP) crystals, which appear shorter than MSU crystals and are often rhomboidal. Under a polarizing filter, CPP crystals change color depending upon their alignment relative to the direction of the red compensator. They are positively birefringent, appearing blue when aligned parallel with the slow axis of the compensator and yellow when perpendicular.
In crystal arthritis, the white blood cell (WBC) count in the synovial fluid is usually 10,000-70,000/µL. However, it may be as low as 1000/µL or as high as 100,000/µL.
Even in the presence of crystals in the joint fluid, blood cultures are indicated if any sign of systemic toxicity is present. Septic arthritis can occur in patients with active crystalline arthropathy.
Gouty attacks are not related to serum levels of uric acid. Thus, an elevated serum uric acid level does not prove the diagnosis of acute gout, though hyperuricemia is present in 95% of cases, and a normal level does not exclude the diagnosis. Renal uric acid excretion should be measured in high-risk patients, including those with renal calculi, a strong family history of gout, and a first attack before age 25 years.
Pseudogout attacks can be triggered by many metabolic abnormalities. Thus, patients who have an initial attack of arthritis with CPP crystals should have a workup that includes a chemistry screen; serum magnesium, calcium, and iron levels; and thyroid function tests.
The WBC count in peripheral blood is usually elevated, with a left shift during acute attacks. The erythrocyte sedimentation rate (ESR) usually is elevated during acute attacks.
Imaging studies of the affected joint or joints are indicated. Patients with new onset of acute gout usually have no radiographic abnormalities. In established disease, radiographs may reveal punched-out erosions or lytic areas with overhanging edges.
Magnetic resonance imaging (MRI) is capable of detecting crystal deposits but is not part of any routine evaluation for acute arthritis. MRI can be very useful in determining the extent of the disease and may help in the differential diagnosis.
Patients with pseudogout usually have degenerative joint changes evident on imaging studies. In addition, they may have calcifications in the soft tissues, tendons, or bursae.
Synovial Fluid Analysis
When a patient presents with acute inflammatory monoarticular arthritis, aspiration of the involved joint is critical to rule out an infectious arthritis and to attempt to confirm a diagnosis of gout or pseudogout on the basis of identification of crystals (see the image below). Minute quantities of fluid in the shaft or hub of the needle are sufficient for synovial fluid analysis.
Analysis of synovial fluid for crystals should ideally be done within 24-48 hours after collection. Synovial fluid specimens may be stored at room temperature without any preservative, but refrigeration (at 4°C/39°F) and ethylenediaminetetraacetic acid (EDTA) preservation is reasonable. A systematic literature review by Meyer et al found that monosodium urate (MSU) crystals were generally stable over time, independent of preservative and temperature, whereas calcium pyrophosphate (CPP) crystals deteriorated over time and were more stable if refrigerated. Re-examining an initially negative synovial fluid sample at 24 hours facilitated detection of additional cases. [112]
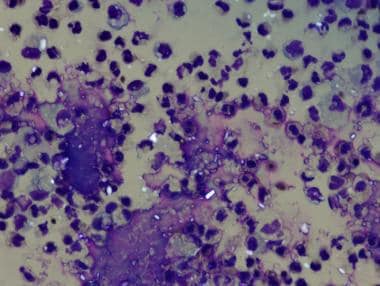
Urate crystals are shaped like needles or toothpicks with pointed ends (see the first image below). Under polarizing light microscopy, urate crystals are yellow when aligned parallel to the axis of the red compensator and blue when aligned across the direction of polarization (ie, they exhibit negative birefringence). Finding negatively birefringent urate crystals (see the second image below) firmly establishes the diagnosis of gouty arthritis.
 Gout. Strongly negative birefringent, needle-shaped crystals diagnostic of gout obtained from acutely inflamed joint.
Gout. Strongly negative birefringent, needle-shaped crystals diagnostic of gout obtained from acutely inflamed joint.
Pseudogout crystals (CPP) are rod-shaped with blunt ends and are positively birefringent. Thus, pseudogout crystals are blue when aligned parallel to the slow ray of the compensator and yellow when they are perpendicular.
A case report by Niessink et al describes the presence of positively birefringent crystals, in the synovial fluid from a swollen and painful joint in a patient with chondrocalcinosis, that Raman spectroscopy identified as calcium carbonate rather than CPP. These authors suggest considering calcium carbonate when positively birefrigent crystals are encountered. [113]
Crystals must be distinguished from birefringent cartilaginous or other debris. Debris may have fuzzy borders and may be curved, whereas crystals have sharp borders and are straight. As alkalization reduces uric acid crystal solubility and the enzyme uricase can “dissolve” these crystals, reduction by addition of sodium hydroxide or uricase to suspected gout crystal can be helpful.
Corticosteroids injected into joints have a crystalline structure that can mimic either MSU or CPP crystals. They can be either positively or negatively birefringent.
The sensitivity of a synovial fluid analysis for crystals is 84%, with a specificity of 100%. If gout remains a clinical consideration after negative analysis findings, the procedure can be repeated in another joint or with a subsequent flare. Crystals may be absent very early in a flare.
Although the sensitivity of this test is inferior, aspiration of synovial fluid from previously inflamed joints that are not currently inflamed may reveal urate crystals. Such crystals are generally extracellular.
Synovial fluid should also be sent for cell count. During acute attacks, the synovial fluid is inflammatory, with a WBC count higher than 2000/µL (class II fluid) and possibly higher than 50,000/µL, with a predominance of polymorphonuclear neutrophils, though low WBC counts are occasionally found.
Synovial fluid glucose levels are usually normal, whereas they may be depressed in septic arthritis and occasionally in rheumatoid arthritis. Measurement of synovial fluid protein has no clinical value.
Crystalline arthritis and infectious arthritis can coexist. Indeed, infectious arthritis is more common in previously damaged joints, which may occur in patients with chronic gouty arthritis. Consequently, in patients with acute monoarticular arthritis, send synovial fluid for Gram stain and culture and sensitivity.
The pathologic specimens must be processed anhydrously. MSU is water-soluble and dissolves in formalin; therefore, only the ghosts of urate crystals may be seen if formalin is used. Absolute (100%) alcohol–fixed tissue is best for identification of urate crystals.
Once a diagnosis of gout is established by confirmation of crystals, repeat aspiration of joints with subsequent flares is not necessary unless infection is suggested or the flare does not respond appropriately to therapy for acute gout.
Serum Uric Acid
Measurement of serum uric acid is the most misused test in the diagnosis of gout. The presence of hyperuricemia in the absence of symptoms is not diagnostic of gout. In addition, as many as 15% of patients with symptoms from gout may have normal serum uric acid levels at the time of their attack. Thus, the diagnosis of gout can be missed if the joint is not aspirated. Remember that situations that decrease uric acid levels can trigger attacks of gout. In such cases, the patient’s medical records may reveal prior elevations of uric acid.
Approximately 25% of the population has a history of elevated serum uric acid, but only a minority of patients with hyperuricemia develop gout. Thus, an abnormally high serum uric acid level does not indicate or predict gout. As noted, gout is diagnosed by the presence of urate crystals in the synovial fluid or soft tissues. More important, some patients who present with a hot swollen joint and an elevated serum uric acid level in fact have infectious arthritis, which may be mismanaged if their synovial fluid is not examined.
Asymptomatic hyperuricemia generally should not be treated. However, patients with levels higher than 11 mg/dL (0.65 mmol/L) and overexcretion of uric acid are at increased risk for renal stones and renal impairment; therefore, kidney function should be monitored in these individuals. [38]
The level of serum uric acid does correlate with the risk for developing gout. The 5-year risk for developing gout is approximately 0.6% if the level is below 7.9 mg/dL, 1% if it is 8-8.9 mg/dL, and 22% if it is higher than 9 mg/dL.
Urinary Uric Acid
A 24-hour urinary uric acid evaluation is generally performed if uricosuric therapy is being considered. If patients excrete more than 800 mg of uric acid in 24 hours while eating a regular diet, they are overexcretors and thus overproducers of uric acid. These patients (approximately 10% of patients with gout) require allopurinol instead of probenecid to reduce uric acid levels. Furthermore, patients who excrete more than 1100 mg in 24 hours should undergo close monitoring of kidney function because of the risk of stones and urate nephropathy.
In patients in whom probenecid is contraindicated (eg, those with a history of renal stones or renal insufficiency), a 24-hour urine test of uric acid excretion need not be performed, because the patient clearly will need allopurinol.
Blood Studies
Blood studies may reveal abnormalities associated with gout or common comorbid conditions. In addition, abnormal results on kidney or liver function studies may affect the selection of therapy.
Obtaining an accurate measure of the patient’s kidney function before deciding on therapy for gout is important. The glomerular filtration rate (GFR) can be estimated by using formulas such as the Modification of Diet in Renal Disease (MDRD) Study equation or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Serum creatinine evaluation alone can underestimate kidney dysfunction in elderly patients or in patients with low muscle mass.
The WBC count may be elevated in patients during the acute gouty attack, particularly if it is polyarticular. Hypertriglyceridemia and low levels of high-density lipoprotein (HDL) are associated with gout. Glucose measurement is useful because patients with gout are at increased risk for the development of diabetes mellitus.
Pseudogout attacks can be triggered by many metabolic abnormalities. Thus, patients who have an initial attack of arthritis with CPP crystals should have a workup that includes a chemistry screen; serum magnesium, calcium, iron and iron-binding levels; and thyroid function tests.
Radiography
Plain radiographs may show findings consistent with gout, but these findings are not diagnostic. Early in the disease, radiographs are often normal or show only soft-tissue swelling. Radiographic findings characteristic of gout, which generally do not appear within the first year of disease onset, consist of punched-out erosions or lytic areas with overhanging edges (see the image below). Haziness suggestive of tophi can be seen in late gout, and tophi may calcify.
Erosions with overhanging edges generally are considered pathognomonic for gout but also can be found in amyloidosis, multicentric reticulohistiocytosis, and type IIA hyperlipoproteinemia. Characteristics of erosions that are typical of gout but not of rheumatoid arthritis include the following:
-
Absence of periarticular osteopenia
-
Location outside the joint capsule
Another characteristic of erosions typical of gout is sclerotic borders, sometimes called cookie-cutter or punched-out borders. In addition, erosions in gout may be distributed asymmetrically among the joints, with strong predilection for distal joints, especially in the lower extremities (see the images below).
Ultrasonography
At the first attack, sites affected with gout may be anechoic on ultrasonography. Later, diffuse enhancement may be evident on the articular cartilage surface. [116] Chondrocalcinosis show up as a thin, hyperechoic band within hyaline cartilage and punctuated pattern on fibrocartilage.
Ultrasonographic findings in established gout include the following [117, 118, 119] :
-
A “double-contour” sign, consisting of a hyperechoic, irregular line of MSU crystals on the surface of articular cartilage overlying an adjacent hyperechoic bony contour
-
“Wet clumps of sugar,” representing tophaceous material, described as hyperechoic and hypoechoic heterogeneous material with an anechoic rim
-
Bony erosions adjacent to tophaceous deposits
The double contour sign is 85% sensitive and 80% specific for crystalline arthritis in general, with specificity for gout of 64% and for calcium pyrophosphate deposition disease of 52%. [120] The reliability of the double contour sign varies with the joint: femoral condyle sensitivity and specificity are 42% and 100%, respectively, compared with 62% and 98% for first metatarsals. [121]
Ultrasonography may demonstrate urate crystal deposition in tissues of asymptomatic patients with hyperuricemia. Pineda et al found double-contour signs in the first metatarsal-phalangeal joints of 25% of 50 asymptomatic patients with hyperuricemia but in none of 52 normouricemic subjects. [122]
Ultrasonography has proved accurate and reliable for detecting calcium pyrophosphate crystal deposition (CPPD). [123] In a study by Ottaviani et al, ultrasonography had higher sensitivity than radiography for detection of CPPD: In 51 patients, ultrasonography revealed hyperechoic spots in all 25 patients with CPPD (sensitivity 100%, specificity 92.3%), whereas radiography revealed CPPD in 16 of the 25 (sensitivity 64%, specificity 100%; P < 0.0001). [124]
In a study by Forien et al that included 32 patients with CPPD and 26 controls, the sensitivity of wrist ultrasonography for the diagnosis of CPPD was 94% while that of wrist radiography was 53.1%; the specificity of ultrasonography was 85%, versus 100% for radiography. [125] Ultrasonography revealed chondrocalcinosis in 35 joints with no radiographic evidence of chondrocalcinosis, and x-rays showed chondrocalcinosis in 3 joints without ultrasonographic chondrocalcinosis, thus indicating the complementary benefit of utilizing both techniques.
In a study by DeMiguel et al, ultrasonography identified urate crystal deposition in 11 of 26 patients who had asymptomatic hyperuricemia for 2-28 years (average, 6.2 years), affecting the knee in nine cases and the first metatarsal-phalangeal joint in six. These results document that asymptomatic gout may not be as innocuous as was once believed. [126]
Ultrasonography has good sensitivity and specificity for detecting tendon involvement, which occurs frequently in gout. In a controlled study by Ventura-Rios et al, which included 80 patients with gout, intra-tendinous tophi were found in tendon insertions at the distal patella, quadriceps, Achilles, and proximal patella. [127]
Computed Tomography
Plain radiography and computed tomography (CT) are complementary for recognizing erosions in gout. [128] Dual-energy CT, using a renal stone color-coding protocol, assesses chemical composition, labeling urate deposits in red. [129] In a case report, Ward et al describe the use of dual-energy CT to diagnose tumoral calcium pyrophosphate crystal deposition, differentiating it from gouty tophus or soft-tissue malignancy. [130]
In a study comparing CT imaging versus a history of urinary tract calculus for identification of nephrolithiasis in gout patients, 62% of the patients with CT-documented scans had no history of urolithiasis. In 383 male patients with primary gout, CT scanning confirmed nephrolithiasis in 103 (26.9%), whereas the history of urinary tract calculus was positive in only 65 (17%). The authors concluded that the prevalence of urolithiasis cannot be accurately determined on the basis of patients’ histories. [131]
Stamp et al reported that multi-energy spectral photon counting computed tomography (SPCCT) was able to detect, differentiate, and quantify monosodium urate crystal deposits in a gouty finger ex vivo, as well as to specifically detect, identify, and quantify calcium pyrophosphate within an osteoarthritic meniscus, and distinguish them from hydroxyapatite crystal deposits. These authors propose that SPCCT has the potential to be useful in diagnosing crystal arthropathies. [132]
Magnetic Resonance Imaging
MRI is not part of any routine evaluation for acute arthritis. MRI evidence of edema is minimal in gout, unless concomitant osteomyelitis is present. [133] However, MRI with gadolinium is recommended when tendon sheath involvement must be evaluated and when osteomyelitis is in the differential diagnosis. Large deposits of crystals may be seen in bursae or ligaments. MRI examination of erosions reveals tophi but no bone edema or synovitis. [134]
Tophi usually have low or intermediate signal intensity on T1-weighted spin echo images. Signal intensity also tends to be low on T2-weighted images. In the absence of inflammation, the tophi are sharply delineated. Presence of inflammation results in increased perilesional signal intensity. Tophi and the surrounding area of inflammation enhance with gadolinium. [135]
In a study of 10 cadaveric knees by Abreu et al, radiographic imaging and histologic analysis demonstrated widespread CPPD crystal deposition in four of the specimens (40%), while MRI demonstrated some calcifications only within the articular cartilage of the femoral condyles in three of those four specimens. In all four specimens, radiographs and histologic analysis had higher sensitivity than MRI. [136]
Histology
Chronic tophaceous gouty deposits frequently show large pale pink acellular areas, which represent dissolved urate crystals, surrounded by histiocytes and multinucleated giant cells (see the image below).
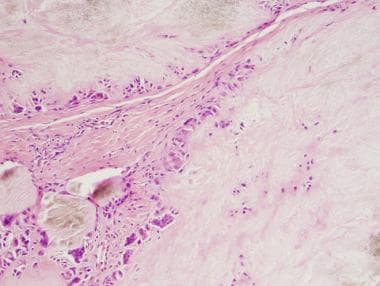 Gout. Hematoxylin and eosin (H&E) stain, low power, showing abundant pale pink areas surrounded by histiocytes and multinucleated giant cells.
Gout. Hematoxylin and eosin (H&E) stain, low power, showing abundant pale pink areas surrounded by histiocytes and multinucleated giant cells.
The crystals are water-soluble and thus are dissolved during routine tissue processing. If there are a large number of crystals, however, some may survive processing and appear as pale brown-gray refractile material (see the image below), or they may be seen on unstained sections. The urate crystals are easily seen on polarized light.
 Gout. H&E stain, high power, showing that most urate crystals have been dissolved but that some pale brown-gray crystals did survive processing.
Gout. H&E stain, high power, showing that most urate crystals have been dissolved but that some pale brown-gray crystals did survive processing.
Pseudogout also demonstrates pale pink areas that may be surrounded by histiocytes and multinucleated giant cells. On higher-power views, however, the crystals are purple and rhomboid and therefore can be distinguished from gout on routine histology (see the images below).
 H&E stain, medium power, of pseudogout with pale pink fibrocartilage in upper portion and purple crystals of calcium pyrophosphate in lower portion.
H&E stain, medium power, of pseudogout with pale pink fibrocartilage in upper portion and purple crystals of calcium pyrophosphate in lower portion.
-
Gout. Acute podagra due to gout in elderly man.
-
Gout. Tophaceous deposits in ear.
-
Gout. Tophaceous deposits on elbow.
-
Gout. Chronic tophaceous gout in untreated patient with end-stage renal disease.
-
Gout. Fluid obtained from tophaceous deposit in patient with gout.
-
Gout. Strongly negative birefringent, needle-shaped crystals diagnostic of gout obtained from acutely inflamed joint.
-
Gout. Plain radiograph showing typical changes of gout in first metatarsophalangeal joint and fourth interphalangeal joint.
-
Gout. Plain radiograph showing chronic tophaceous gouty arthritis in hands.
-
Gout. Radiograph of erosions with overhanging edges.
-
Gout. Needles of urate crystals seen on polarizing microscopy.
-
Gout. Hematoxylin and eosin (H&E) stain, low power, showing abundant pale pink areas surrounded by histiocytes and multinucleated giant cells.
-
Gout. H&E stain, high power, showing that most urate crystals have been dissolved but that some pale brown-gray crystals did survive processing.
-
Pseudogout. H&E stain, high power, under polarized light to highlight rhomboidal crystals.
-
H&E stain, medium power, of pseudogout with pale pink fibrocartilage in upper portion and purple crystals of calcium pyrophosphate in lower portion.
-
Pseudogout. H&E stain, high power, of calcium pyrophosphate crystals, demonstrating their rhomboidal structure.
-
What is gout? Gout is an inflammatory disease where uric acid precipitates into crystals that deposit in various joints around the body, causing pain and inflammation. This video describes the pathophysiology, causes, symptoms, and treatment of gout.
-
This wrist radiograph shows chondrocalcinosis of the radiocarpal joint (arrow).
-
The most common site of calcium pyrophosphate disease (CPPD) involvement, as seen on radiography, is the patellofemoral joint, followed by the radiocarpal joint (shown). Classic radiographic findings are chondrocalcinosis (yellow arrow), hooked osteophytes, and subchondral cysts (red arrow).